Ernest Rutherford's Gold-Foil Experiment
Scientific experimentAbout
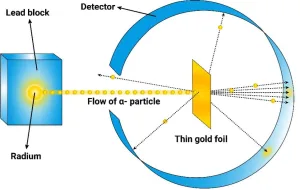
Ernest Rutherford's Gold-Foil Experiment was a pivotal scientific study conducted in 1911, which significantly altered the understanding of atomic structure. Rutherford, assisted by Hans Geiger and Ernest Marsden, fired alpha particles at a thin sheet of gold foil. The experiment aimed to test the prevailing "plum pudding" model of the atom, proposed by J.J. Thomson, which suggested that atoms are composed of a diffuse positive charge with electrons embedded within. However, the results showed that most alpha particles passed through the gold foil with minimal deflection, while a small fraction were deflected at large angles, and some even bounced back. These findings led Rutherford to propose the nuclear model of the atom, where the positive charge and most of the mass are concentrated in a small nucleus at the atom's center, surrounded by a vast empty space containing electrons. This model revolutionized atomic theory by introducing the concept of a dense nucleus, contrasting sharply with the earlier diffuse charge model. The experiment's impact was profound, laying the groundwork for further research into atomic structure and the development of quantum mechanics. It remains a cornerstone in the history of physics, highlighting the power of experimental science in challenging and transforming established theories.