Ernest Rutherford's Gold Foil Experiment
Scientific experimentAbout
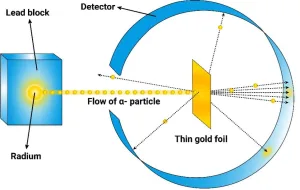
Ernest Rutherford's Gold Foil Experiment was a pivotal scientific study conducted between 1908 and 1913. The experiment involved firing positively charged alpha particles through a thin sheet of gold foil to understand the structure of atoms. Prior to this, the prevailing model was J.J. Thomson's plum pudding model, which suggested that atoms were composed of a uniform positive charge with negative electrons embedded within. Rutherford's setup included a phosphorescent screen to detect where the alpha particles landed after passing through the gold foil. The results were groundbreaking. Most alpha particles passed straight through the gold foil, indicating that atoms are mostly empty space. However, some particles were deflected at large angles, and a few even bounced back, suggesting a small, dense, positively charged nucleus at the atom's center. This led Rutherford to propose the nuclear model of the atom, replacing the plum pudding model. The nuclear model posits that atoms consist of a small nucleus surrounded by electrons in circular orbits, revolutionizing our understanding of atomic structure.